Describe the Characteristics of a Polar Molecule
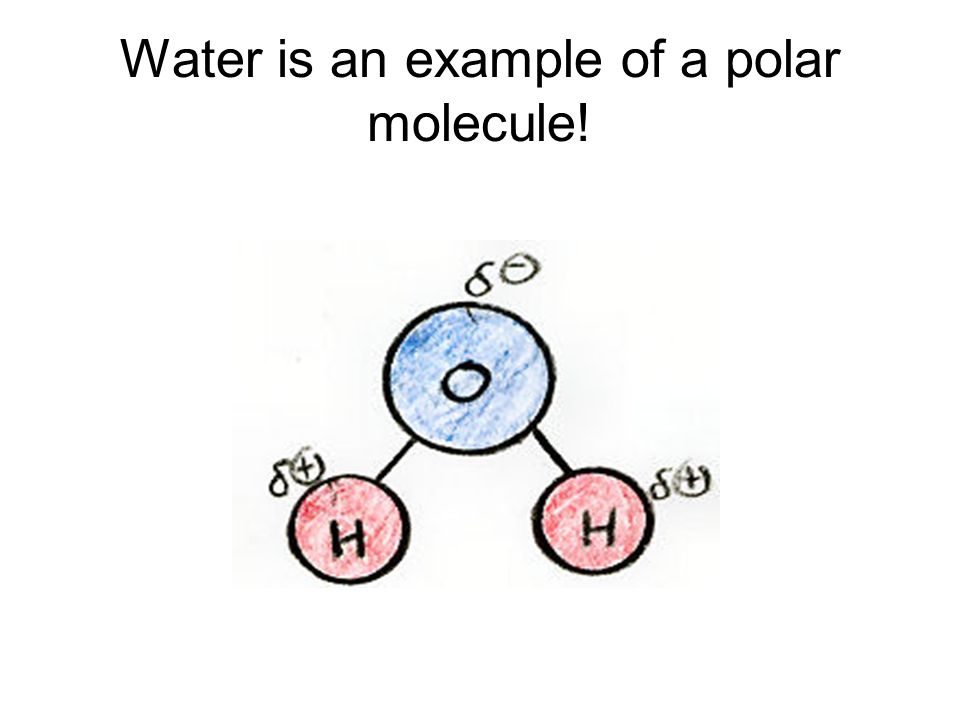
Water or H2O is an example of a polar. O Dissociation is the electrostatic attraction between an ion and a polar molecule.
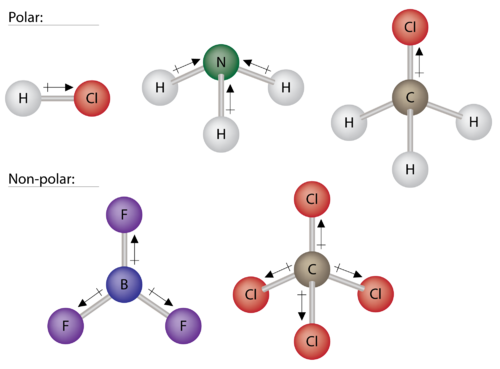
Polar Molecules Chemistry For Non Majors
Water H 2 O is a polar molecule.

. Molecule with an unequal distribution of charge resulting in the molecule having a positive end and a negative end element any substance that cannot be broken down into simpler substances. The bond angle between the sulfur atom and the two chlorine atoms is 1095 since the two vectors are of equal intensity but their directions do not coincide. If they are added vectorially they do not cancel each other out.
A polar molecule is characterized by the uneven distribution of the electrons that form the covalent bonds between each atom in the molecule resulting in a slightly positively charged side and a slightly negatively charged side. The non polar molecule is electrically neutral and stable. In a polar molecule one side of the molecule has a positive electrical charge and the other side has a negative electrical charge.
A molecule that has a net dipole moment due to its having unsymmetrical polar bonds. The oxygen side of the molecule has a slight negative charge while the side with the hydrogen atoms has a slight positive charge. The mass of a moleculethe molecular mass The mass of a molecule which is the sum of the masses of its atoms.
The resulting molecule is considered polar where oxygen becomes slightly negative and the hydrogen becomes slightly positive. No charge separation can be observed in the case of nonpolar molecules. Complete the paragraph to describe the characteristics of a borane molecule BH3.
Select the correct answer below. Polar molecules tend to dissolve well in water and other polar solvents. Polar molecule is characterized by an uneven distribution of the electrons that form the covalent bonds between each atoms in the molecule.
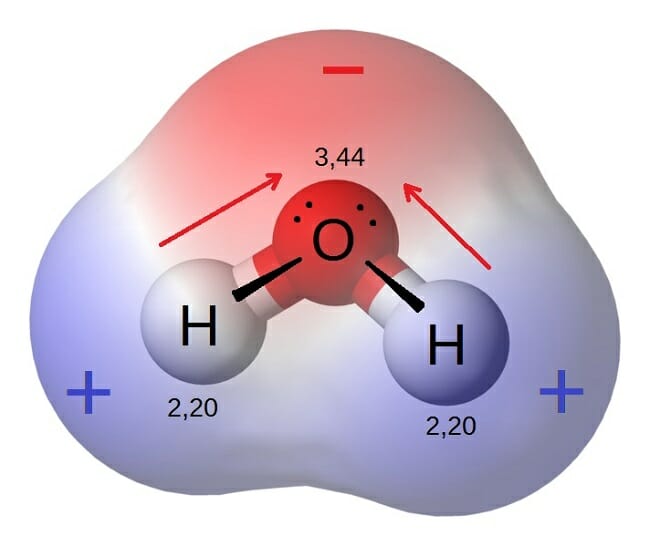
Characteristics of polar molecule. Unequal sharing of electrons makes water a polar molecule. - they should have opposite charge.
These bonds are said to be polar bonds because of the difference in electronegativity between the atoms. Polar molecules have partial positive and partial negative charges within the molecule. Analysis of it shows that the sequence of atoms in this molecule is C.
The molecule is. 1 on a question Water is referred to as a polar molecule. The bond polarities in BH3 are polar or nonpolar the molecular shape is trigonal pyramidal or trigonal planar or tetrahedral or linear or bent and the molecule is polar or.
The process of theses cells are talked and learnt in science. Water is referred to as a polar molecule. This leads to the hydrogen being slightly positive and the oxygen being slightly negative we denote this as δ.
Some characteristics of polar molecules are discussed below. Explain the conditions under which this molecule could cross by simple diffusion. Describe the characteristics of a polar molecule.
You might be interested in. Describe the characteristics of a polar molecule. Nonpolar molecules have low boiling and melting points.
This occurs because of the differences in electronegativity between atoms of different elements. Molecules can also be non polar. When 2 atoms are close to each other in electronegativity then they share electrons equally.
Oxygen is much more electronegative than hydrogen and thus the electrons within the H-O bond tend to spend more time around the O. Examples include O 2 N 2 and F 2 You can use the following chart to predict the type of bond. For example water is.
Its a polar molecule. Complete the paragraph to describe the characteristics of a sulfur dichloride SCI molecule. As a result an sci molecule is.
Describe the characteristics of lonic electrolytes Question Which of the following best describes the term dissociation. I would describe the characteristics of the tail of a phospholipid molecule would be mosaic of lipid molecules. Given their geometry and the difference in electronegativity between chlorine and sulfur they are molecules with a marked polar character.
As with formula masses it is important that you keep track of the number of atoms of each element in the molecular formula to obtain the correct molecular mass. The Lewis structure and table of electronegativities are given. There are also amphiphilic molecules large molecules that have both polar and nonpolar groups attached to them.
The Lewis structure and table of electronegat. The Lewis structure and table of electronegativities are given. Electrons are unequally shared and theyre drawn to one nucleus more than the other opposite charges are separated at 2 ends of a bond Hydrogen Bond.
JeleCi-5-ci An SCl2 molecule has bonds and a molecular shape. - they should have same dipole moment value. The bonds between hydrogen and oxygen are distributed so that the hydrogen atoms are both on one side of the oxygen atom rather than evenly spaced.
Polar molecules have high boiling and melting points. 3 Describe the characteristics of a molecule that could cross a cell membrane by protein mediated active transport. 3 They should have electronegativity difference.
Describe the characteristics of a molecule that could cross a cell membrane by facilitated diffusion. Complete the paragraph to describe the characteristics of a borane molecule BH3. Sometimes called the molecular weightis simply the sum of the masses of its atoms.
- they should have electronegativity different. O Dissociation is the extent to which a covalent solute may be dissolved in a solvent. 1 Polar molecules should have opposite charge.
2 They should have some dipole moment value. A polar molecule is a type of molecule that has a separation of electric charge where one side of the molecule is positively charge and the other side is negatively charged.

Polar Covalent Bond Definition And Examples
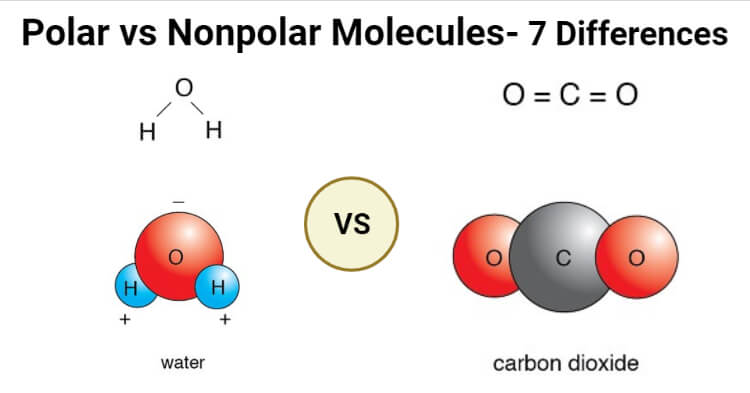
Polar Vs Nonpolar Molecules Definition 7 Key Differences Examples

Polar Molecules Chemistry For Non Majors
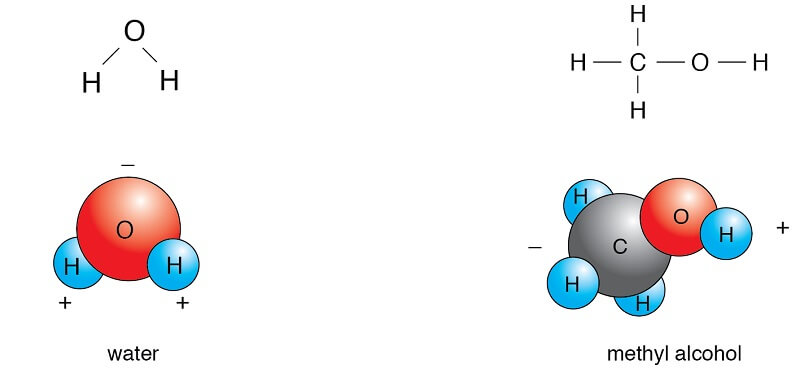
Polar Vs Nonpolar Molecules Definition 7 Key Differences Examples

Polar And Nonpolar Covalent Bonds Definitions Molecules And Examples
Difference Between Polar And Nonpolar Molecules Definition Formation Properties Examples
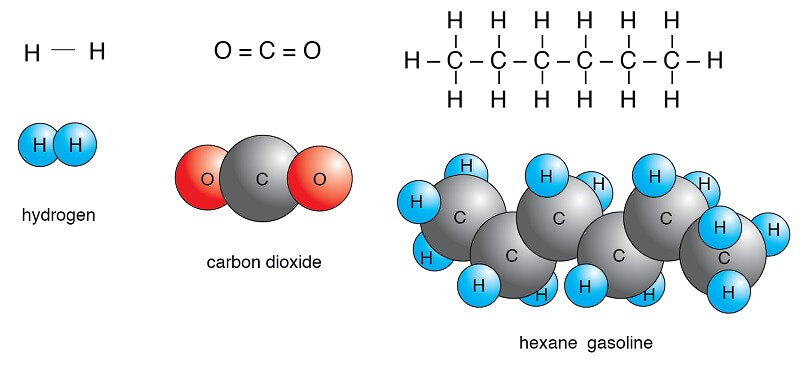
Polar Vs Nonpolar Molecules Definition 7 Key Differences Examples

Why Is Water Considered A Polar Molecule Quora

What Is Polarity Definition Example Polar Vs Non Polar Molecules
Polar Bonds And Molecules Ppt Download

Polar Molecule Characteristics Example What Makes A Molecule Polar Video Lesson Transcript Study Com

Polar Molecule Characteristics Example What Makes A Molecule Polar Video Lesson Transcript Study Com

Properties Of Water I Will Define Characteristics Of Polar And Non Polar Molecules I Can Describe The Characteristics Of Acids And Bases I Will Define Ppt Download

What Is Polarity Definition Example Polar Vs Non Polar Molecules
Difference Between Polar And Nonpolar Molecules Definition Formation Properties Examples

A Permanent Dipole Permanent Dipole Or Keesom Forces They Exist Download Scientific Diagram
Polar Molecule Definition And Examples Biology Dictionary

Polar Molecules Chemistry For Non Majors

Polar Molecule Characteristics Example What Makes A Molecule Polar Video Lesson Transcript Study Com
Comments
Post a Comment